Pfizer’s Lipitor strategy worked… pretty well!
Posted: February 24, 2012 Filed under: Strategy Leave a commentIt’s been almost 3 months since Pfizer’s Lipitor lost exclusivity so it’s not a bad time to assess how the company’s strategy of maintaining market share has worked so far. Keep in mind that Pfizer really broken new ground with this strategy. Most R&D-based pharmaceutical companies practically abandon all sales and marketing efforts once a drug loses patent protection since within a month or two almost all of their market share is wiped out by the lower priced generics. However, Lipitor is not your typical drug as shown by its $10B+ per year revenue numbers. If Pfizer could keep even 10% of that market share, they would have a revenue stream that a lot of pharmaceutical companies would kill for.
Before we look at the numbers, how about a quick primer on how the generics market works? When an R&D-based pharmaceutical company first gets a new (small-molecule) drug approved, it’s via a New Drug Application (NDA). Based on either the patents around the new drug, or the market exclusivity awarded through the NDA, the company has the sole right to sell the drug. The logic behind this right is that it allows a company to recoup the costs associated with R&D over a defined period of time. The period of exclusivity ends when a generics company gets an ANDA (Additional New Drug Application) approved after the patent “runs out” (or sometimes before it runs out by proving the patent is invalid).
Now here is the important part. The ANDA has its own period of exclusivity. The first generics company to get an ANDA approved gets 180-days of exclusivity as the sole provider of a generic alternative to the branded drug. Again, the logic behind this is to provide a financial incentive to offset the costs of getting an ANDA approved and that incentive is substantial. During the 180-day period, the price of the drug drops only 10-20% (so the generic manufacturer gets almost the same profit margin as the branded-manfucturer did, but only for 180 days). Once that 180-day period of exclusivity runs out, any generics company can get an ANDA approved and sell the drug, thus competition drastically increases and drug prices drop to maybe 10-30% of the branded drug’s price. At this point, profit margins are razor-thin and the drug is basically a commodity. Due to the rather steep price cuts that come along with generics, the brand name drug typically loses all of its market share within a month or two of the first generic hitting the market, as most brand name manufacturers have little interest in competing on price.
In the case of Lipitor, Ranbaxy was awarded with the first ANDA approval and with a little help from Teva, they were able to overcome some manufacturing (and regulatory) difficulties and got their generic version of Lipitor to the market just after Pfizer’s last patent ran out. The other generic was a so-called “authorized generic”, which is in fact a generic version of Lipitor produced under the approval of Pfizer (which the rules allow). That version is produced by Watson and Pfizer gets a pretty nice slice of that pie as a result of the arrangement (70% of revenues according to this article).
Now with all the background out of the way, how has Pfizer’s strategy fared so far? Pretty good. As of mid-February, Pfizer still has approximately 41% market share of all atorvastatin prescriptions. If we run some rough numbers based on Lipitor sales for 2010 ($10.7B), a 41% market share would bring in over $2B in revenue for the 180-day ANDA exclusivity period (the only time Pfizer has a chance of keeping market share). Lipitor had been slowly losing market share even before the patent expired, so let’s assume a more conservative $1.5B. All of the effort that Pfizer put into keeping market share (PBM contracts, co-pay cards) doesn’t come cheap, so let’s knock the figure down to $1B. However, Pfizer’s cut of sales from Watson’s authorized generic (which by some simple math has about 20% of the market) probably pushes that up to $1.25B.
Not bad at all! Rather than leaving Lipitor to the generics companies, Pfizer spent a little time and money and has successfully held onto a sizeable chunk of the market and gets to put another $1B or so in the bank. A wise investment by any stretch of the imagination.
What will be interesting to see is if any of the other R&D-based pharmaceutical companies follow suit. There are some big drugs going off patent in 2012 (Seroquel, Plavix, Singulair) and Pfizer may have just proven that a little effort can provide some big pay offs. Keep a look out for more stories like this in the coming year!
UPDATE (3/15/2012): Adam Fein over at Drug Channels just put up a great post about Pfizer’s Lipitor strategy and it has some more recent data. I suggest you check it out!
Has pharma been doing R&D wrong all these years?
Posted: January 4, 2012 Filed under: Innovation, Strategy 3 CommentsYou can’t keep up on the latest biopharmaceutical industry news without hearing about the crisis in R&D productivity. Basically, the amount of money being spent on R&D by companies has been growing at a rapid pace, but so far productivity, as measured by the number of new products that reach the market, has not been keeping up. In fact, it’s been pretty flat over the last 20 or so year as the graph below illustrates (link to source). As a result, the cost of getting a new drug approved by the FDA has been pegged at north of $1B when you include all the money spent on projects that go nowhere.
What’s the reason behind his trend? Well, a number of theories have been put forth:
1. All the “low hanging fruit” have been picked. I honestly find this reason to be pretty weak. Sure, we probably don’t need another opioid receptor agonist/antagonist and I doubt there is much use in finding another M1-muscarinic receptor agonist, but considering how little we know about the biological systems that make up the human body I find the idea that we’re running out of easy targets laughable. There are plenty of easy targets out there that would produce a plethora of new drugs, the problem is we don’t know what they are.
2. The strategic shift from away from innovation to financial returns has killed productivity. There is likely something to this point. However, this trend didn’t just start happening in the mid-1990s. I was recently speaking with a gentleman who is getting close to retirement after spending most of his life in pharma. He mentioned how in the mid-1970s he was working for Searle and a new CEO came in who completely thrashed R&D in order to improve the bottom line. I have no doubt that a strategy that is overly focused on profit maximization can reduce R&D productivity, but I don’t think that’s the root cause of today’s problems.
3. The recent spat of mergers and acquisitions has killed morale among R&D personnel. Again, I think there is something to this, but mergers and acquisitions aren’t a recent phenomenum either. I personally witnessed the level of morale at a big pharmaceutical company during the multiple mergers that happened in the early 2000s and yes, productivity took a nose dive. However, I don’t think this is the root cause of the drop in R&D productivity.
At this point you might be asking “Well, what is it then?” I don’t claim to have the final answer, but a recent paper in Nature Reviews – Drug Discovery gave me pause because it backed up a hypothesis that I’ve been thinking about for the past few years.
The most logical way to approach this problem is to ask the question: When were things better and what has changed since then? If we look back to the golden age of pharma, say the 1950s – 1970s, we see incredible productivity. Entire drugs classes were discovered during this time including the benzodiazepines, antipsychotics, synthetic opiods, antifungals, etc, etc. Janssen Pharmaceuticals alone discovered over 70 new chemical entities over a 40 year period starting in the 1950s, with many of those occurring in the earlier years.
So what changed since then? Well, the Nature paper discusses the shift in R&D strategy from phenotypic-screening to target-based screening. In layman’s terms, it was the change from screening drugs based on the response they produced in living tissue or an organism to screening drugs based on the effect they produced on a drug target (typically a receptor or enzyme). Phenotypic-screening is how the benzodiazepines were discovered. The first benzodiazepine, chlordiazepoxide, was not made because they thought it would make for a good anxiety drug, it was an unexpected product that was produced during a chemical reaction. The chemical was then administered to a laboratory animal (likely a mouse or rat) and the sedative effect was noted.
Contrast this with the drug discovery strategy that is typically seen today in pharmaceutical companies: researchers isolate a drug target that is believed to play a role in a disease and then the chemists and biologists go about making new chemicals that interact with the receptor in a particular way, optimizing for solubility, logP and all the other metrics that make for a good drug. At this point they have no idea if the drug actually works, they only know it interacts with the target. They then move to animal models of the disease and try to confirm efficacy, which if successful leads to the drug being tested in humans.
The key difference between these two drug discovery strategies is that the first (phenotypic-screening) ignores how the drug works and just focuses on if it works. Target-based screening focuses on knowing how the drug works, not if it works (yes, I’m painting with a broad brush here, but bear with me). Now, if you’re in the business of discovering new drugs that interact with biological systems that you have little understanding of, which makes more sense as a strategy? Which is more likely to lead you to the discovery of a new class of drugs? It’s really a choice between trying to expand on current knowledge (target-based screening) and throwing a hail-mary and trying to find something you never knew existed (phenotypic screening).
If you’re at all interested in this topic, I strongly encourage you read the Nature paper (it’s open-access) and look at some of the data that the authors uncovered. They do a much better job of explaining the trade offs between the two methods and came up with some pretty interesting evidence that the shift away from phenotypic-screening has had direct consequences on R&D productivity.
Maybe it’s time for pharma to look to the past for guidance on how to suceed in the future?
More Lipitor news: Ahhh… that’s why independent pharmacists are mad!
Posted: December 5, 2011 Filed under: Strategy Leave a commentYet another updated on the Lipitor-is-going-generic saga. Rather than reiterating what I said in prior blog posts, I’ll just add some interesting tidbits of information.
Over at the drug distribution blog DrugChannels (highly recommended), Adam Fein posted some very interesting commentary on the Lipitor story. When the news about Pfizer’s agreements with major PBMs to get preferential treatment for Lipitor, even after the generics became available (in some cases the PBM wouldn’t reimburse for the generic at all), a group called Pharmacists United for Truth and Transparency (PUTT) had this to say:
The statement called the move “a blatant attempt” by benefit managers to keep Pfizer’s discount while employers still have to pay the full price of the brand-name drug.
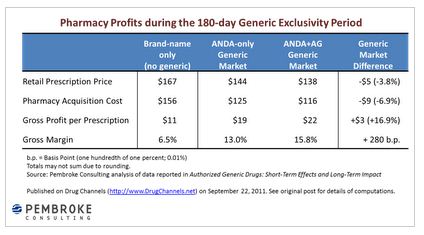
Hmmm…. that’s awfully heartwarming that a group of pharmacists decided to look out for employers who offer drug coverage. However, if you dig a little deeper, you’ll see there is a “healthy dose of economic self-interest” in play here, as outlined in Adam Fein’s blog. What is of particular interest is this chart Adam put together…
Now things become a little clearer! PUTT says they are outraged that employers will be stuck paying higher drug prices if Lipitor is used instead of the generic (which, by the way, they have no proof of), but I would hazard to guess that some of their anger comes from the fact that they are missing out on those juicy margins they usually make during the 180-day exclusivity period.
If you want to see how contentious this issue of pharmacy margins can be, check out the comment section of a another blog post by Adam here. Who knew drug distribution strategy could elicit such emotion?
Update: Pfizer and Lipitor (Show me the money!)
Posted: November 19, 2011 Filed under: Strategy Leave a commentI posted a few days ago about Pfizer’s strategy for dealing with the upcoming loss of exclusivity for it blockbuster drug Lipitor. Well, there is more news on that front, this time concerning the Federal Trade Commission’s (FTC) interest in the deals Pfizer made with pharmacy benefit managers (PBMs).
As reported by Pharmalot, the FTC has started calling around, asking about the details of the contracts. It’s not an official investigation yet, but does show that the federal government is concerned that Pfizer is participating in anti-competitive practices. However, if you read the letters from the PBMs to pharmacies that were leaked, it does appear that Pfizer (through the use of discounts) is making the continued use of branded Lipitor the cheapest option out there. If you check out page three of the link, you’ll see that Catalyst Rx is being offered a 31% discount, which bring the cost of Lipitor for the insurance company to just under what generic Lipitor would cost in the first six months after the patent expires.
In addition, it appears that my guess that Medco was passing on all of the discount to the insurance provider was correct. Coventry (which relies on Medco to manage its pharmacy benefits) had this to say…
A Coventry spokesman confirms the deal was cut directly with Pfizer. “Most of Coventry’s fully-insured members will save money on Lipitor when we pass on the savings by lowering their pharmacy co-pay to the amount they would pay for the generic. We think our members will appreciate the change and lower co-pays, but it also reduces our bottom-line cost of Lipitor, which helps Coventry keep coverage more affordable.” He declined, though, to offer any specifics.
So it would appear that Pfizer is isn’t doing anything underhanded at all. They are providing discounts which makes Lipitor the lowest cost option, even after generic become available.
However, the question remains, why is Pfizer doing this? Is it simply to cash in on another 6 months of Lipitor profit (albeit at a lower margin and smaller overall market)? That could be, but I would guess that this the first stage of a multistage strategy to keep Lipitor profits flowing (or at least a significant portion of them).
As I mentioned in my previous post, keep an eye out in the next 3-4 months for news about Lipitor and Pfizer. If there is a long-term plan involved, Pfizer should start rolling out the next stage soon.
What on earth is Pfizer up to with Lipitor?
Posted: November 15, 2011 Filed under: Strategy Leave a commentNow it’s not everyday that you see something this unexpected in the pharmaceutical industry, so I have to comment.
It’s old news at this point, but Pfizer’s Lipitor, the best selling drug in the history of the industry, will lose its market exclusivity on November 30, 2011. At it’s peak, Lipitor had annual sales of over $13 billion dollars, so this is no minor event. A lot of people in the industry were wondering how Pfizer would deal with this and it appears we’re getting a sneak preview of their strategy.
Today, a number of news agencies reported on a letter that Medco Health Solutions (one of the largest pharmacy benefit managers in the US) sent to a number of pharmacies. The letter basically stated “…even though generic Lipitor will become available, keep filling prescriptions with Lipitor from Pfizer.” Now why on earth would they do that? Typically, as soon as drugs lose their market exclusivity (usually because a patent expires) generic drugs flood the market and the price drops very quickly. Why would Medco want pharmacies to keep prescribing the more expensive, branded version of Lipitor? Well, if we read the news reports, we quickly find out why…
“Pfizer has agreed to large discounts for benefit managers that block the use of generic versions of Lipitor, according to a letter from Catalyst Rx, a benefit manager for 18 million people in the United States.”
Get out the pitchforks!!! Pfizer is colluding with pharmacy benefit managers (PBMs) to keep generics out! Just another example of multinational corporations, drunk with power, steamrolling the little guy, right? At least that’s what it sounds like if you listen to Geoffrey F. Joyce, an associate professor of pharmaceutical economics and a health policy expert at the University of Southern California…
“This is just an egregious case. Clearly there’s been some negotiation between Pfizer and the large P.B.M.’s saying we’re going to make this cost-beneficial to them, but the plan sponsors are going to eat it.”
OK, before you get too worked up, a quick lesson on PBMs. PBMs are hired by insurance companies and by employers with health plans to manage their drug expenses. In fact, they compete with each other, trying to provide the best plans they can. That’s why it seems like your prescription drug benefit changes every year when its time to re-enroll in your employer’s health plan. Do you really think Medco is going to negotiate an agreement where Pfizer gives them a discount on Lipitor and they pocket the entire thing? Hardly.
What’s happening here is Pfizer has negotiated with the PBMs and agreed to a rebate structure that basically undercuts the yet-to-arrive generic versions of Lipitor. Although the prices of generic drugs are less than the branded price, they typically only drop 20-30% in the first six months because the first generic version gets 6 months exclusivity (they are the only one who can sell generic Lipitor). After the 6 months of exclusivity are up, the drug basically becomes a commodity and the price drops 80-90% compared to the branded version. What Pfizer is doing here is providing some pretty hefty discounts to the PBMs (probably 20-30%), undercutting the generic companies and making branded Lipitor the most attractive option from a price perspective. Those hefty rebates that the PBMs negotiated? They aren’t keeping them. That money will filter through to the insurance providers and employer-sponsored plans. If it didn’t, Medco would be in a world of hurt.
Now that we have that figured out, the more interesting question is “What on eath is Pfizer up to with Lipitor?” There has to be some strategy behind this because once the 6 month generic exclusivity runs out, the price is going to drop even more and Pfizer is unlikely to agree to a 80-90% discount. There has been talk of an over-the-counter (OTC) version of Lipitor coming out, but that would take over a year to get approval from the FDA.
All I can say is, keep an eye on Pfizer. They are up to something and it’s a strategy that the pharmaceutical industry has never seen before.
How NOT to commit Medicare fraud…
Posted: November 2, 2011 Filed under: Strategy Leave a commentLast week, Amgen announced their third quarter earnings which included a $780 million charge related to a legal settlement they entered into with the federal government and a number of states concerning “illegal sales and marketing practices”. Now $780 million dollars is a large amount of money by anyone’s measure, but if you read the third quarter report, it sounds like there were some “questionable” actions on the part of Amgen, but it’s all worked out now and we can get on with business, right?
Wrong.
If you read the Massachusetts whiteblower lawsuit court documents, it appears that Amgen committed some very serious crimes which weren’t the action of just one or two employees, but rather an intentional marketing tactic that involved a large number of Amgen employees all the way up to senior management. And if you live in the US and pay taxes, you should be very, very mad.
The product in question is Amgen’s Aranesp, a erythropoetin analogue, which stimulates the production of red blood cells in the body. It is used in patients undergoing chemotherapy (since many anti-cancer drugs damage bone marrow, where red blood cells are made) and patients with chronic kidney disease (since erythropoetin is made in the kidneys) to boost their levels of red blood cells. It’s a very profitable drug for Amgen, with total sales just north of $2.5 billion dollars in 2010. Now this is where things get a little complicated… Aranesp is a longer-acting version of another Amgen product, Epogen, the first erythropoetin analog that was originally used for dialysis patients. When Epogen was developed, Amgen outlicensed the rights to Epogen, outside of use in dialysis, to J&J, which sells it’s version as Procrit. So as you can imagine Amgen’s current strategy: get patients who use Epogen or Procrit to use Aranesp, since why would you want to share the market with J&J?
Now that we have the background laid out, we can get down to the details of the case. Aranesp is sold in either vials (as pictured above) or as prefilled-syringes. Since it’s impossible to to get all of the liquid out of a vial, or inject all of the liquid in a syringe, manufacturers of injectable drugs often “overfill” their products, or basically add a little bit more of the drug to the vial or syringe, so that when a physician or patient administers the drug, they get the full dose. How much do they need to overfill? Less than 10% typically.
Well, someone at Amgen had a smart idea that they could overfill the vials or syringes of Aranesp by more than 10%, then “hint, hint, nudge, nudge”, let doctors know that they are actually getting more drug than what they paid for. Why would they do that you ask? Well, doctors can bill insurance companies and Medicare for each dose of Aranesp they give patients (they actually bill in 5ug increments). So if a doctor orders 5 vials of Aranesp (each one overfilled by 20%), they can actually bill for the 6 doses of Aranesp they actually gives patients (basically pooling the extra 20% until it adds up to a full dose). Amgen was giving physicans a kick-back for using Aranesp, paid for by YOU, the taxpayer (in the case of Medicare/Medicaid patients).
Now you might be thinking this was a scheme devised by a few rogue Amgen employees, working on their own accord. Well, you’d be wrong. Nevermind the fact that sales reps don’t have any control over how much “overfill” is included in each vial or syringe of Aranesp (that’s a decision made by manufacturing), there is also the fact that information on how much the vials were overfilled and how much extra money physicans could make charging insurance companies for the overfill amount was sent out by Amgen’s medical affairs department (document below is from this link).
And to top it all off, it appears that Amgen’s CEO, Chris Sharer, knew about the overfill scheme the entire time. An email from a Senior Manager of Medical affiars reads as follows…
… an email dated January 6, 2006 from Edwin Mar, Senior Manager of Medical Information, to Helen Torley, Vice President and General Manager of Nephrology, and Leslie Mirani, Vice President of Sales, states: “In regards to your request to provide EPOGEN overfill historical information to [CEO] Kevin Sharer, these are the information I have available so far regarding EPOGEN 1.0 mL vial fill volumes.” The email goes on to provide the overfill amounts for Epogen from 1993 through January 2006:
(1993-Q4/2002) – 1.168 mL
(Q4/2002-Q1/2004) – 1.144 mL
(Q1/2004 – present) – 1.111 mL
Yup, you read that right, the older erythropoetin analog that Amgen wanted patients to stop using? They started to reduce the overfills in that product to ensure that doctors were only getting a kick-back for Aranesp.
I’m literally at a loss for words. The pharmaceutical industry has taken a lot of heat (rightly so) for their off-label marketing of drugs and other nefarious activities, but this takes the cake. This is not some sales rep promoting a drug for an unapproved condition (in that case, at least the patient is getting what they paid for), this is a deliberate, organized scheme to defraud both private insurance companies, Medicare/Medicaid and in the end, you, the American taxpayer. Honestly, I think anyone associated with this scheme, from the CEO down to the account managers needs to be fired and the whole organization rebuilt, from the ground up. I mean, how else do you rid a company of the mentality that thinks this is OK?
In the future, prescription drug prices will be lower than you might think
Posted: October 13, 2011 Filed under: Strategy Leave a commentThis is something I’ve been thinking about for a while. The cost of health care is rising everywhere, but it seems to be a particularly critical issue in the US. While the cost of drugs is a approximately 10% of total health care spending in the US, it is increasing, albeit more slowly than total health care spending.
This is related to a previous post, where I commented on how it seems like every pharmaceutical and biotech company is jumping on the oncology bandwagon. One of the big reasons for this trend is that the pricing pressure on oncology drugs is relatively low. Insurance companies consider oncology relatively “untouchable”, that is, telling a mother of three that you won’t pay for her cancer drugs will cost you far more in terms of public relations than it will ever cost in terms of dollars. This is why we now have drugs like Yervoy ($120K/treatment) and Provenge ($93K/treatment). And these high prices aren’t limited to cancer. There are drugs that cost over $10,000/year in areas such as asthma, multiple sclerosis and lupus, to name just a few. Is this strategy really sustainable? The answer is no, it’s not. But what will stop this trend of ever increasing drug prices?
The answer is you. You’ll simply stop paying for these drugs.
You’re probably thinking “Come on! I never pay full price for my drugs! The worst case scenario is that I pay a $25 or $50 co-pay, not my problem!” That’s probably true right now, but might not be true in the future if the trend towards high deductible health plans (HDHP) continues. The number of Americans covered by HDHPs has more than doubled since 2008 and continues to rise (see chart below). Why? The premiums on HDHPs are often a fraction of what HMO or PPO coverage is. It not only reduces costs for your employer, but it also reduces the premiums that are deducted off your paycheck every month.
What are HDHPs? Well, under typical HMO or PPO coverage, you only pay a co-pay on your prescription drugs. Depending on if it’s a generic or a branded drug, your co-pay might be $5, $10 or even $50, but that’s all you’ll pay whether or not the drug’s actual cost is $5 or $5000. Under a HDHP, you pay the full amount of your drug costs, up to the annual deductible limit (the limit includes all health care expenses). These plans are almost always paired with a health care saving account (HSA), where you (or your employer) make tax-free contributions that you can use to pay for your out of pocket expenses.
So let’s say you visit your physician and he says “Mr. Smith, it looks like you’ve got a bacterial infection, might be serious, let’s put you on Zithromax.” Under the HMO or PPO plan, you might say, “Sounds good doc! Thanks!” and then run to your pharmacy and complain about the $25 co-pay. Under the HDHP plan, you’ll likely repsond “Really? How much is Zithromax? $200?!?!? Is there anything else I could take that isn’t so expensive? Amoxicillan? $4 at Walmart? Thanks Doc!!”. Of course, this isn’t a likely scenario if you have cancer, but for non-life threatening diseases, it’s a conversation I could see happening.
As medical costs in general, and prescription drug costs in particular, continue to be shifted towards the patient, there will be an increased awareness on the part of patients of the costs of drugs and choices will have to be made, hopefully through consultation with physicians.
So what does this mean for drug companies? The strategy of shifting from $200/month drugs for large patient populations, to $10,000/month drugs for smaller patient populations, won’t work forever. Eventually patients will start to say “This is coming out of my pocket, is there anything cheaper?” and drug companies will be facing real pricing pressure, across the board.
It’s a challenging situation to be in for drug companies since producing safe, effective drugs is an expensive process. However, eventually another strategy (lower priced drugs) will have to replace the current one. That’s going to require more than just a shift in strategy by the drug companies, it will require a complete reappraisal of how patients, physicans, insurance companies, the FDA and drug companies view value and risk.
I’ll be honest and say I have no idea what the drug industry will look like in 10 or 20 years. However, I do know that the drug companies that will be on top are the ones planning for this future right now.
More news on the obesity front!
Posted: October 12, 2011 Filed under: Latest news Leave a commentAmazing! No less than 10 days after I blogged about Vivus resurrecting their NDA for Qnexa, Orexigen sent out a press release detailing their plan to resubmit their NDA for their obesity drug Contrave.
To give you a little bit of background, the FDA rejected Orexigen’s NDA, despite a majority vote to approve (13 to 7), due to concerns about cardiovascular safety. The FDA ended up “recommending” (I put it in quotes since nothing the FDA recommends is actually optional) that another clinical trial be run to eliminate concerns over over an increase in blood pressure seen during the phase III clinical trials that Orexigen submitted as a part of thier original NDA.
Originally, this caused Orexigen’s leadership to announce they were abandoning Contrave. Details are scarce, but apparently the FDA requested a clinical trial design that was so out of touch with what a company like Orexigen could muster that the company decided it wasn’t even worth discussing.
However, after a period of negotiations with the FDA, Orexigen apparently reached a deal where they could resubmit their NDA if they conduct a cardiovascular safety trial comprised of approximately 10,000 patients. If Orexigen considers that a win, I can’t even imagine what the FDA’s original demand looked like.
So now, Orexigen is estimating that they can complete the trial and resubmit their NDA in 2014. Rather than critique the company’s announcement, I’ll just point you to Adam Feuerstein’s article where he points out the utter ridiculousness of Orexigen’s optimism.
If you don’t feel like reading the article, I’ll give you the cliff notes: Orexigen has $70M in the bank and a conservative estimate for the cost of a 10,000 patient trial is $100M. However, Contrave is being co-developed with Takeda who has much deeper pockets than Orexigen. A more insurmountable obstacle is the time line of NDA resubmittal in 2014. A trial that size will take one to two years, just to enroll all 10,000 patients, never mind administer the drug, collect data, resubmit the NDA and get FDA approval.
If Orexigen/Takeda pulls this off, it will be a victory indeed. I just worry it might be a pyrrhic victory.
Vivus vs. the FDA: Round Two
Posted: September 17, 2011 Filed under: Strategy Leave a commentIn an earlier post, I promised to provide updates about the on-going saga to get an obesity drug approved by the FDA and surprisingly, there is some news on that front from Vivus.
Despite the FDA’s rejection of Qnexa back in October of last year, Vivus has decided to go for broke and give it another try. If you recall, Qnexa is a combination of phentermine and toperimate, two drugs that are already FDA-approved. The problem is that mothers who take toperimate during pregancy may have a greater risk of delivering a child with a cleft palate. Due to the anticipated widespread use of any approved obesity drug (including use by pregnant women), the FDA has set very high standards in terms of safety (some would say ridiculously high standards). Needless to say, an obesity drug that causes birth defects would not meet those standards.
In their latest press release, Vivus announced that the FDA has accepted an early resubmission of their NDA based on three recently completed studies…
“Topiramate teratogencity data published and presented since our last meeting with the FDA in April 2011 includes two case-control studies….In addition, a birth defect study from Denmark on newer generation antiepileptic drugs including topiramate was published in JAMA. In all of these studies, the authors concluded that topiramate was not a major teratogen,” commented Wesley Day, vice-president clinical development.”
I wish Vivus all the best, but I’m a little concerned about this resubmission. First off, the quote “the authors concluded that topiramate was not a major teratogen” (the emphasis is mine) doesn’t exactly instill confidence in terms of the drug’s safety for pregnant women and the children they are carrying. However, it appears that Vivus will be pursuing an indication that excludes pregnant women, at least for the initial NDA…
“In this initial indication, we plan to include a contraindication for women of childbearing potential. We believe this is a sound approach that, if approved, will potentially allow early commercialization in a higher-risk population with a significant unmet medical need. The FORTRESS study remains important in our plan to more precisely define the teratogenic potential of topiramate and may enable us to expand the indication to include obese women of child-bearing potential. If the FORTRESS results are favorable, we expect to file for the full indication in late 2012.”
You might be thinking “What’s the problem? They just won’t give it to pregnant women!”
Well, the issue is two-fold:
- Vivus included a contra-indication for pregnant women with their initial NDA and it was rejected. You can say that pregnant women shouldn’t take Qnexa, but it’s difficult to convince the FDA that they won’t. Unless Vivus can institute a very rigid REMS program to ensure that pregnant women won’t have access to the drug, I feel that the FDA is not going to play along. Since the FORTRESS study results are expected in late 2012, and I assume that it is a very comprehensive study of the teratogenicity of toperimate, the FDA may say “Still too risky, let’s just wait until the FORTRESS date comes in.”
- If Vivus does offer an acceptably rigorous REMS program to keep pregnant women from getting access to Qnexa, it will severely curtail the launch of the drug. A rigid enough REMS program will likely mean that only certain doctors can prescribe Qnexa, only certain pharmacies can fill these prescriptions and for each patient who takes the drug, an extensive tracking system will have to instituted to collect all the follow-up safety data. Not exactly conducive to big revenues, now is it?
However, the FDA did agree to the early resubmission, so I assume that the agency sees some validity to Vivus’ data and amended NDA. This story will definitely be worth keeping an eye on and if Vivus is successful, it may pay off handsomely for them, but I feel that they still have a long road ahead of them.
Biosimilars: A little more complicated than we thought…
Posted: September 12, 2011 Filed under: Innovation Leave a commentMost people are familiar with generic drugs and how big the cost savings can be. Typically, once a branded drug goes off patent, the price drops precipitously, usually somewhere in the range of 80-90% (excluding the 180 days of market exclusivity that the first company to produce the generic gets). Now that biologics have become such a large part of the drug market (estimates are the will be the top 6 selling drugs by 2014), the most obvious question to ask is “when will generic versions of biologics be available?”
The answer to that question is important to a lot of people, namely insurance providers who pay tens of thousands of dollars a year for each patient who takes drugs like Avastin, Epogen and Rituxan. The issue is so important in fact, that the Congressional Budget Office conducted a study that estimated that the government could save up to $25 billion dollars over 10 years from the introduction of biosimilars.
The problem was, until the Health Care Reform Act was passed last year, there was no regulatory process to approve biologic drugs. With the passage of the bill, the FDA was given a mandate to create such a regulatory process. Problem solved, right? Not so fast. There are two specific issues that seem to be taking the wind out of the sails for those promoting biosimilars.
The first issue is the approval pathway. Back in August, Janet Woodcock (director of CDER at the FDA) and others published an article in the New England Journal of Medicine outlining the FDA’s view of a potential biosimilar approval pathway (keep in mind that the health care bill simply told the FDA to create a pathway, it didn’t actually lay out how to do it). The article is worth reading, but the best way to summarize it is “We’ll figure it out as we go.” Yikes. Not exactly what companies want to hear when it comes to estimating the risks of investing in the development of a biosimilar. Now, I’m not blaming the FDA for saying what they said; biologic drugs are incredibly complex compared to small molecule drugs (see figure below) and until the FDA has had a chance to “test” the approval pathway, they can’t really come out and say “Do X, Y and Z and we’ll approve you drug.” So what does this mean? For the most part, the only players in the biosimilar market will the big biopharmaceutical companies who have both the expertise and more importantly, financial backing, to forge an approval pathway. Think of the process less like hiking in the Rockies with a compass and more of using a machete to cut a path through the dense jungles of South-East Asia.
This image gives you some idea of just how complex biologics are. On the right is a molecule of aspirin and on the left is an antibody (a number of biologics are antibodies).
The second issue is physician and patient perception of biologic drugs. Small molecule generics are literally the exact same drug as the branded products, at least to the degree that we can tell (which is really well). Not so with biologic drugs. Heck, it’s not uncommon for a company that produces biologic drugs to have difficulty producing consistent batches of them in their own plants. In light of this, physicians will likely be very wary of switching a patient from a branded biologic to a generic, especially since biologics are often used to treat conditions where drug efficacy can be a matter of life or death/debilitation (cancer, MS, etc). In fact, Decision Resources recently release a report where a majority of surveyed physicians said they would hesitate to prescribe a biosimilar for a patient, unless the biosimilar had gone through phase 3 trial for that indication. Ouch. Phase 3 trials are typically the most expensive part of drug development and their costs can easy run into the tens of millions of dollars, if not more. If a company is required to conduct such trials, the likelihood of a biosimilar being 80-90% cheaper than the branded generic is pretty much zero.
Prediction: Once biosimilars hit the market (and they will, eventually), I can almost guarantee that the companies who own the branded biologics will conduct a marketing campaign, targeted at both physicians and patients, where they hammer away at the “similar” part. I can see the commercials now “Biosimilars are not like your generic Lipitor, they are only “similar”, not identical. Do you really want to risk your health on something “similar”?”
So all in all, biosimilars have a pretty rough road ahead. The big biopharmaceutical companies are already making serious investments in the area since there is a lot of money at stake, but it’s not a task for the faint of heart. Most likely we’ll start to see biosimilars of the relatively simpler biologics like the insulins, where the cost of development is more predictable and less costly. Either way, it’s an interesting area to keep an eye on because I have no doubt that there will be some spectacular successes in the area and some spectacular failures.